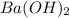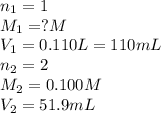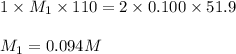Chemistry, 10.01.2020 11:31 mpzpowell7506
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.110 l0.110 l sample of an unknown hno3hno3 solution required 51.9 ml51.9 ml of 0.100 m ba(oh)20.100 m ba(oh)2 for complete neutralization. what is the concentration of the hno3hno3 solution? concentration:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(a...
Questions

Computers and Technology, 15.08.2020 18:01









Advanced Placement (AP), 15.08.2020 18:01


Mathematics, 15.08.2020 18:01





Mathematics, 15.08.2020 18:01



Chemistry, 15.08.2020 18:01

 solution will be 0.094 M.
solution will be 0.094 M.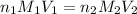
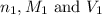 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 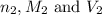 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is