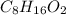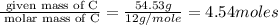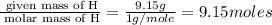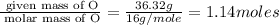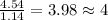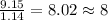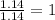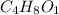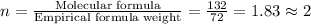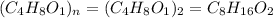Chemistry, 21.10.2019 16:50 arnold2619
Acompound is 54.53% c,54.53% c, 9.15% h,9.15% h, and 36.32% o36.32% o by mass. what is its empirical formula? insert subscripts as needed. empirical formula: chocho the molecular mass of the compound is 132 amu.132 amu. what is its molecular formula? insert subscripts as needed. molecular formula: cho

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Acompound is 54.53% c,54.53% c, 9.15% h,9.15% h, and 36.32% o36.32% o by mass. what is its empirical...
Questions


Mathematics, 13.02.2020 20:23



Mathematics, 13.02.2020 20:23








Spanish, 13.02.2020 20:23

Mathematics, 13.02.2020 20:23


English, 13.02.2020 20:23

Health, 13.02.2020 20:24

Computers and Technology, 13.02.2020 20:24