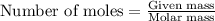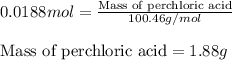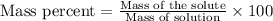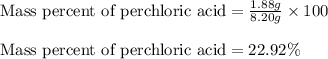Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
A8.20 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. if...
Questions



Advanced Placement (AP), 23.05.2021 01:30


Mathematics, 23.05.2021 01:30





Mathematics, 23.05.2021 01:30


English, 23.05.2021 01:30



English, 23.05.2021 01:30

Mathematics, 23.05.2021 01:30

Mathematics, 23.05.2021 01:30



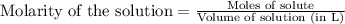
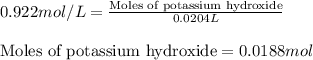
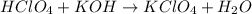
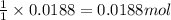 of perchloric acid.
of perchloric acid.