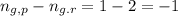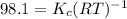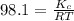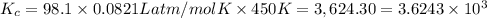Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl3(g)+cl2(g)⇌pcl5(g). a 7.5-l gas vessel is charged with a mixture of pcl3(g) and cl2(g), which is allowed to equilibrate at 450 k. at equilibrium the partial pressures of the three gases are ppcl3 = 0.125atm , pcl2 = 0.155atm , and ppcl5 = 1.90atm kp= 98.1 what is kc?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2

Chemistry, 23.06.2019 13:00
Me puede ayudar con estas preguntas 1. diga que estudia la química orgánica explica el nacimiento bioquímicas 2. cual es la importancia de la química orgánica. 3. determine las principales características del hidrógeno, oxigeno, nitrógeno y azufre como elementos que constituyen los compuestos orgánicas. 4. elabore una tabla comparativa entre compuestas orgánicas e incaicos.
Answers: 1
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl3(g)+cl2...
Questions

Mathematics, 11.12.2019 17:31


Social Studies, 11.12.2019 17:31



Chemistry, 11.12.2019 17:31

Mathematics, 11.12.2019 17:31

Mathematics, 11.12.2019 17:31




History, 11.12.2019 17:31

Mathematics, 11.12.2019 17:31

Biology, 11.12.2019 17:31

Mathematics, 11.12.2019 17:31


Social Studies, 11.12.2019 17:31


Mathematics, 11.12.2019 17:31

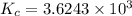 .
.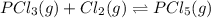
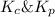 is given by:
is given by: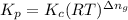
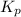 = Equilibrium constant in terms of partial pressure.=98.1
= Equilibrium constant in terms of partial pressure.=98.1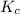 = Equilibrium constant in terms of concentration =?
= Equilibrium constant in terms of concentration =?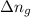 = Difference between gaseous moles on product side and reactant side=
= Difference between gaseous moles on product side and reactant side=