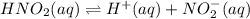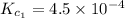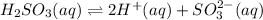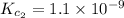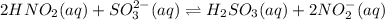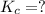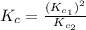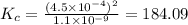Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq)h2so3(aq)⇌⇌h+(aq) + no2−(aq)2h+(aq) + so32−(aq)kc = 4.5 × 10−4kc = 1.1 × 10−9 what is the value of kc for the reaction 2hno2(aq) + so32−(aq)⇌h2so3(aq) + 2no2−(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq...
Questions

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01




Mathematics, 22.10.2020 01:01


Social Studies, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01


Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

English, 22.10.2020 01:01



Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

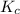 for the final reaction is, 184.09
for the final reaction is, 184.09