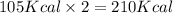Chemistry, 26.06.2019 03:40 ayoismeisjjjjuan
When nh3(g) reacts with n2o(g) to form n2(g) and h2o(g), 105 kcal of energy are evolved for each mole of nh3(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
When nh3(g) reacts with n2o(g) to form n2(g) and h2o(g), 105 kcal of energy are evolved for each mol...
Questions




English, 03.10.2021 14:00



History, 03.10.2021 14:00

Arts, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00

Geography, 03.10.2021 14:00

Chemistry, 03.10.2021 14:00




Arts, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00



Spanish, 03.10.2021 14:00

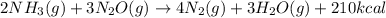
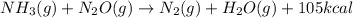
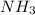 , the coefficient 3 is put before the
, the coefficient 3 is put before the 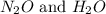 and the coefficient 4 is put before the
and the coefficient 4 is put before the 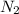 .
.