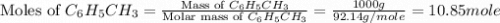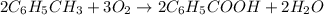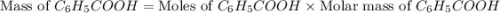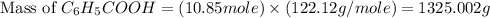Chemistry, 26.06.2019 04:10 samsavage4073
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h, which is used to prepare the food preservative sodium benzoate, c6h5co2na. what is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3


Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2
You know the right answer?
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h...
Questions

Physics, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57



Mathematics, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57


Mathematics, 10.06.2020 13:57


Health, 10.06.2020 13:57

Mathematics, 10.06.2020 13:57

English, 10.06.2020 13:57





Mathematics, 10.06.2020 13:57

Health, 10.06.2020 13:57

English, 10.06.2020 13:57

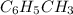 = 1 Kg = 1000 g
= 1 Kg = 1000 g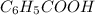 = 122.12 g/mole
= 122.12 g/mole