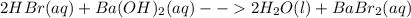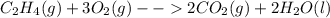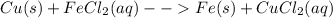Classify each of these reactions. 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) c2h4(g)+3o2(g)⟶2co2(g)+2h2o(l) c2h4(g)+3o2(g)⟶2co2(g)+2h2o(l) cu(s)+fecl2(aq)⟶fe(s)+cucl2(aq) cu(s)+fecl2(aq)⟶fe(s)+cucl2(aq) na2s(aq)+2agno3(aq)⟶2nano3(aq)+ag2s (s) na2s(aq)+2agno3(aq)⟶2nano3(aq)+ag2s (s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Classify each of these reactions. 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) 2hbr(aq)+ba(oh)2(aq)⟶2h2o(...
Questions

English, 17.04.2020 22:55

Mathematics, 17.04.2020 22:55




Health, 17.04.2020 22:55



Chemistry, 17.04.2020 22:55

History, 17.04.2020 22:55






Mathematics, 17.04.2020 22:55

History, 17.04.2020 22:55

Arts, 17.04.2020 22:55


Business, 17.04.2020 22:55