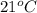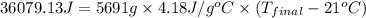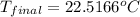Chemistry, 26.06.2019 05:10 kaitlan225
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c...
Questions




Mathematics, 30.10.2020 21:10




Mathematics, 30.10.2020 21:10

Mathematics, 30.10.2020 21:10

Mathematics, 30.10.2020 21:10



Mathematics, 30.10.2020 21:10

Mathematics, 30.10.2020 21:10


Biology, 30.10.2020 21:10




Mathematics, 30.10.2020 21:10

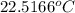
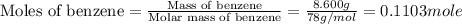
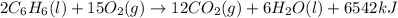
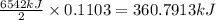 of energy on combustion.
of energy on combustion.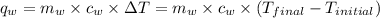
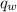 = heat released = 360.7913 kJ = 36079.13 J
= heat released = 360.7913 kJ = 36079.13 J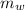 = mass of water = 5691 g
= mass of water = 5691 g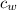 = specific heat of water=
= specific heat of water= 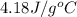
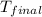 = final temperature = ?
= final temperature = ?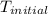 = initial temperature =
= initial temperature =