
Chemistry, 26.06.2019 16:10 pchisholm100
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° = ? use the reactions here to determine the δh° for reaction (i):
(ii) hcl(g) ⟶ hcl(aq) δh(ii) ° = −74.8 kj
(iii) h2 (g) + cl2 (g) ⟶ 2hcl(g) δh(iii) ° = −185 kj
(iv) alcl3 (aq) ⟶ alcl3 (s) δh(iv) ° = +323 kj/mol
(v) 2al(s) + 6hcl(aq) ⟶ 2alcl3 (aq) + 3h2 (g) δh(v) ° = −1049 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
Questions



Biology, 29.10.2019 06:31


Social Studies, 29.10.2019 06:31





Physics, 29.10.2019 06:31

Engineering, 29.10.2019 06:31


Mathematics, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31

Chemistry, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31


Mathematics, 29.10.2019 06:31

Mathematics, 29.10.2019 06:31

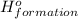 for the reaction is -1406.8 kJ.
for the reaction is -1406.8 kJ.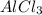 is:
is: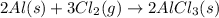
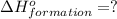
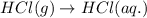
 ( × 6)
( × 6)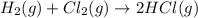
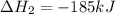 ( × 3)
( × 3)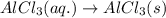
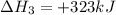 ( × 2)
( × 2)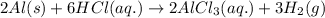
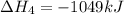
![\Delta H^o_{formation}=[6\times \Delta H_1]+[3\times \Delta H_2]+[2\times \Delta H_3]+[1\times \Delta H_4]](/tpl/images/0020/0754/79ecd.png)
![\Delta H^o_{formation}=[(-74.8\times 6)+(-185\times 3)+(323\times 2)+(-1049\times 1)]=-1406.8kJ](/tpl/images/0020/0754/37adf.png)


