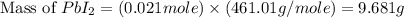The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(aq)⟶pbi2(s)+2nac lo3(aq) what mass of precipitate will form if 1.50 l of concentrated pb(clo3)2 is mixed with 0.200 l of 0.210 m nai? assume the reaction goes to completion. mass of precipitate:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(a...
Questions


Mathematics, 31.03.2020 05:01

History, 31.03.2020 05:01

English, 31.03.2020 05:01








History, 31.03.2020 05:01

Mathematics, 31.03.2020 05:01

Mathematics, 31.03.2020 05:02

Mathematics, 31.03.2020 05:02

Mathematics, 31.03.2020 05:02


Mathematics, 31.03.2020 05:02

English, 31.03.2020 05:02

History, 31.03.2020 05:02

 precipitate produced will be, 9.681 grams.
precipitate produced will be, 9.681 grams. .
.
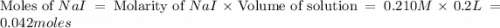

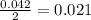 moles of
moles of