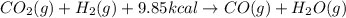Chemistry, 26.06.2019 20:20 Santos7446
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each mole of co2(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
When co2(g) reacts with h2(g) to form co(g) and h2o(g) , 9.85 kcal of energy are absorbed for each m...
Questions

Mathematics, 12.07.2019 14:20






Mathematics, 12.07.2019 14:20




Chemistry, 12.07.2019 14:20

Biology, 12.07.2019 14:20


Mathematics, 12.07.2019 14:20





History, 12.07.2019 14:20