
Chemistry, 27.06.2019 03:20 xnadertheking
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the reaction of 10.0 g of na2o2 with water is kj. 2na2o2 (s) + 2h2o (l) → 4naoh (s) + o2 (g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the react...
Questions


Mathematics, 06.01.2021 04:20

Advanced Placement (AP), 06.01.2021 04:20

Mathematics, 06.01.2021 04:20

Mathematics, 06.01.2021 04:20









Social Studies, 06.01.2021 04:20

SAT, 06.01.2021 04:20


Mathematics, 06.01.2021 04:20


History, 06.01.2021 04:20

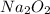 will be -8.064 kJ.
will be -8.064 kJ.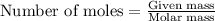

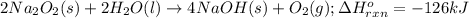
 of energy.
of energy.

