
Chemistry, 27.06.2019 09:10 alleshia2007
If 58.67g of mercuric oxide were completely decomposed to generate 54.34 g of mercury how many grams of oxygen should have been produced

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
If 58.67g of mercuric oxide were completely decomposed to generate 54.34 g of mercury how many grams...
Questions

English, 30.11.2021 02:30



Computers and Technology, 30.11.2021 02:30



Computers and Technology, 30.11.2021 02:30


English, 30.11.2021 02:30

Mathematics, 30.11.2021 02:30


Health, 30.11.2021 02:30








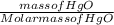
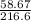 = 0.271mol
= 0.271mol

