Chemistry, 27.06.2019 10:10 Chatoloko231
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how many moles are present in this sample?
when answering the question, include the following:
state how to find the molar mass for the hydrocarbon.
state how you know if you need to multiply or divide by the molar mass.
give the correct number of significant figures and explain why the answer has that many significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2

Chemistry, 23.06.2019 19:40
How many atoms of each element are in the chemical formula p205
Answers: 1
You know the right answer?
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how m...
Questions





Social Studies, 15.07.2020 18:01


Social Studies, 15.07.2020 18:01

Mathematics, 15.07.2020 18:01



Computers and Technology, 15.07.2020 18:01

History, 15.07.2020 18:01



Mathematics, 15.07.2020 18:01



Mathematics, 15.07.2020 18:01


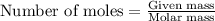
![[(2\times 12)+(6\times 1)]=30g/mol](/tpl/images/0022/9571/19171.png)