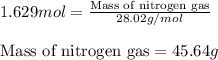Chemistry, 27.06.2019 19:30 38saferguson
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n204 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 92.02 g/mol, n2h4 32.05 g/mol n204) 2 n2h4(1)3 n2(g) + 4 h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions


History, 16.07.2019 20:20

Mathematics, 16.07.2019 20:20

History, 16.07.2019 20:20

Mathematics, 16.07.2019 20:20

English, 16.07.2019 20:20







Mathematics, 16.07.2019 20:30



Mathematics, 16.07.2019 20:30



Social Studies, 16.07.2019 20:30

Biology, 16.07.2019 20:30

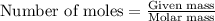 .....(1)
.....(1)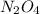
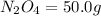
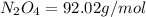
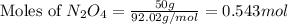
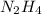
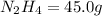
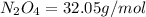
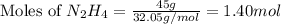
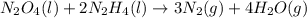
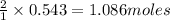 of
of 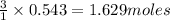 of nitrogen gas.
of nitrogen gas.