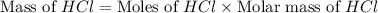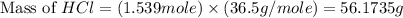Chemistry, 27.06.2019 19:30 helpmeplz11239
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to the following balanced reaction. a possibly useful molar mass is bc13 117.16 g/mol. bc13(g)+3 h20(1) -- h3bo3(s)+3 hc1(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3

Chemistry, 23.06.2019 11:20
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2
You know the right answer?
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to...
Questions





English, 10.10.2019 01:30

Chemistry, 10.10.2019 01:30

Biology, 10.10.2019 01:30




Mathematics, 10.10.2019 01:30

English, 10.10.2019 01:30

Spanish, 10.10.2019 01:30

Biology, 10.10.2019 01:30



English, 10.10.2019 01:30


History, 10.10.2019 01:30

Mathematics, 10.10.2019 01:30

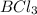 = 60 g
= 60 g
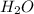 = 37.5 g
= 37.5 g
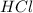 = 36.5 g/mole
= 36.5 g/mole
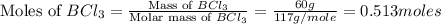
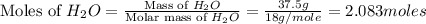
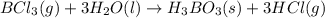
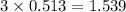 moles of
moles of