
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper sulfate pentahydrate is 249.68 g/mol, calculate grams of copper sulfate pentahydrate that will dissolve in 100 g of water at 0oc (show calculations for full credit)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Given that the density of the saturated solution is found to be 1.16 g/ml. the molar mass of copper...
Questions

Mathematics, 03.06.2020 14:00

Biology, 03.06.2020 14:00

History, 03.06.2020 14:00


Mathematics, 03.06.2020 14:00

Chemistry, 03.06.2020 14:00

Mathematics, 03.06.2020 14:00



Mathematics, 03.06.2020 14:00




History, 03.06.2020 14:00



Mathematics, 03.06.2020 14:00


Social Studies, 03.06.2020 14:00

Mathematics, 03.06.2020 14:00

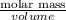
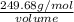
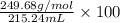
 .
. 

