
Chemistry, 29.01.2020 19:02 Pizzapegasus1
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to water vapor at 110.0 °c. the specific heats of ice, water, and steam are 2.09 j/g-k, 4.18 j/g-k, and 1.84 j/g-k, respectively. for , δhfus = 6.01 kj/mol and δhvap = 40.67 kj/mol.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

You know the right answer?
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to wat...
Questions

English, 13.12.2020 02:20


Mathematics, 13.12.2020 02:20









Chemistry, 13.12.2020 02:20




Mathematics, 13.12.2020 02:20

English, 13.12.2020 02:20


Biology, 13.12.2020 02:20

Physics, 13.12.2020 02:20

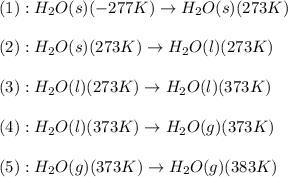
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0482/9975/e4ef0.png)
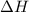 = enthalpy change = ?
= enthalpy change = ?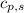 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk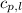 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk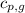 = specific heat of liquid water = 1.84 J/gk
= specific heat of liquid water = 1.84 J/gk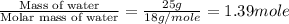
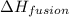 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole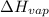 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25g\times 4.18J/gK\times (273-277)k]+1.39mole\times 6010J/mole+[25g\times 2.09J/gK\times (373-273)k]+1.39mole\times 40670J/mole+[25g\times 1.84J/gK\times (383-373)k]](/tpl/images/0482/9975/b0606.png)
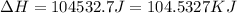 (1 KJ = 1000 J)
(1 KJ = 1000 J)

