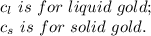Chemistry, 28.06.2019 18:30 ilizzy1224
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg of liquid gold at an initial temperature at 1336k. the solid and liquid other. when the two reach thermal equilibrium will the mixture be entirely solid, or will they be in a mixed solid/liquid phase? explain how you know. draw two separate temp. vs. energy added diagrams to you answer this question. can only exchange heat with each

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
2.0 kg of solid gold (au) at an initial temperature of 1000k is allowed to exchange heat with 1.5 kg...
Questions

Mathematics, 25.11.2020 19:00







Mathematics, 25.11.2020 19:00

Arts, 25.11.2020 19:00






Mathematics, 25.11.2020 19:00