
Chemistry, 28.06.2019 20:20 joseroblesrivera123
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) 2zn (s) + h+ (aq) → 2zn2+ (aq) + h2 (g) zn (s) + 2hbr (aq) → znbr2 (aq) + 2h+ (aq) zn (s) + 2br- (aq) → znbr2 (aq) zn (s) + 2hbr (aq) → znbr2 (s) + 2h+ (aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is the net ion...
Questions

Health, 02.10.2020 15:01



Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

English, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Physics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

English, 02.10.2020 15:01

History, 02.10.2020 15:01


History, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01


Social Studies, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Health, 02.10.2020 15:01

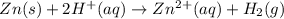
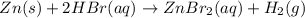
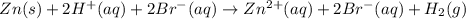
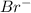 ion is the spectator ions.
ion is the spectator ions.


