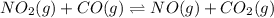Chemistry, 28.06.2019 21:20 tamikalove78
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume of the system is decreased at constant temperature, what change will this cause in the system? a shift to produce more no a shift to produce more co a shift to produce more no2 no shift will occur

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume...
Questions


History, 30.07.2019 00:30







Computers and Technology, 30.07.2019 00:30

Computers and Technology, 30.07.2019 00:30


Computers and Technology, 30.07.2019 00:30




Computers and Technology, 30.07.2019 00:30


Computers and Technology, 30.07.2019 00:30

Social Studies, 30.07.2019 00:30