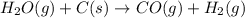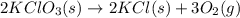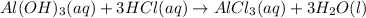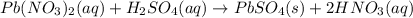Chemistry, 29.06.2019 01:20 ambernolinan
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co(g) + h2 (g) (b) 2kclo3 (s) ⟶ 2kcl(s) + 3o2 (g) (c) al(oh)3 (aq) + 3hcl(aq) ⟶ alcl3 (aq) + 3h2 o(l) (d) pb(no3 )2 (aq) + h2so4 (aq) ⟶ pbso4 (s) + 2hno3 (aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

You know the right answer?
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co...
Questions








Mathematics, 13.07.2021 20:30



Mathematics, 13.07.2021 20:30


Mathematics, 13.07.2021 20:30


English, 13.07.2021 20:30

Mathematics, 13.07.2021 20:30




Computers and Technology, 13.07.2021 20:30