
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon tank of gasoline is burned in an automobile engine. assume that gasoline is primarily octane, c8h18,c8h18, and that the density of gasoline is 0.692 g⋅ml−1.0.692 g⋅ml−1. this assumption ignores additives. also, assume complete combustion. co2co2 released:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

You know the right answer?
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon...
Questions


Biology, 10.04.2020 20:30

Mathematics, 10.04.2020 20:30


Physics, 10.04.2020 20:30

History, 10.04.2020 20:30

Physics, 10.04.2020 20:30





Mathematics, 10.04.2020 20:30



Mathematics, 10.04.2020 20:30

Mathematics, 10.04.2020 20:30




Mathematics, 10.04.2020 20:30

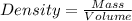
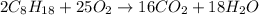
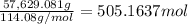
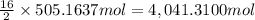 of carbon-dioxide
of carbon-dioxide

