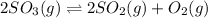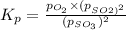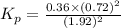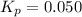Chemistry, 29.06.2019 03:10 cookies1164
Asample of so3 is introduced into an evacuated sealed container and heated to 600 k. the following equilibrium is established: 2 so3( g) ∆ 2 so2( g) + o2( g) the total pressure in the system is 3.0 atm and the mole fraction of o2 is 0.12. find kp

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

You know the right answer?
Asample of so3 is introduced into an evacuated sealed container and heated to 600 k. the following e...
Questions

Social Studies, 11.11.2020 02:40


Mathematics, 11.11.2020 02:40


Arts, 11.11.2020 02:40


Mathematics, 11.11.2020 02:40


Mathematics, 11.11.2020 02:40

Mathematics, 11.11.2020 02:40



Mathematics, 11.11.2020 02:40




Social Studies, 11.11.2020 02:40

Chemistry, 11.11.2020 02:40

Social Studies, 11.11.2020 02:40

English, 11.11.2020 02:40

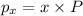
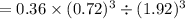
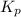 is 0.050.
is 0.050.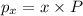
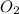 is 0.12
is 0.12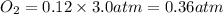
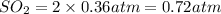 Thus the partial pressure of
Thus the partial pressure of 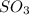 is = [3 - (0.36+0.720)] atm = 1.92 atm
is = [3 - (0.36+0.720)] atm = 1.92 atm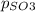 = 1.92 atm
= 1.92 atm