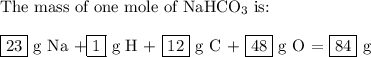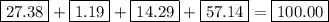Chemistry, 01.07.2019 00:10 lazerlemon500
(need all boxes answered)
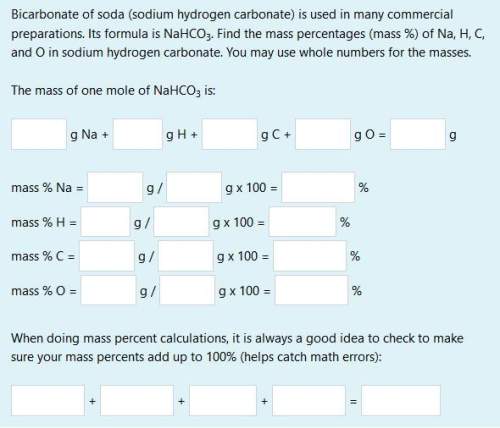
bicarbonate of soda (sodium hydrogen carbonate) is used in many commercial preparations. its formula is nahco3. find the mass percentages (mass %) of na, h, c, and o in sodium hydrogen carbonate. you may use whole numbers for the masses.
the mass of one mole of nahco3 is:

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
Questions




Mathematics, 11.02.2020 16:31




Computers and Technology, 11.02.2020 16:32