Chemistry, 01.07.2019 16:10 cherry12345627
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−850 kj when 12.0 mol of al undergoes the reaction with a stoichiometrically equivalent amount of fe2o3. express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−8...
Questions




Biology, 20.10.2019 04:30

Mathematics, 20.10.2019 04:30

Computers and Technology, 20.10.2019 04:30


Mathematics, 20.10.2019 04:30



Mathematics, 20.10.2019 04:30

Mathematics, 20.10.2019 04:30

Spanish, 20.10.2019 04:30






Physics, 20.10.2019 04:30


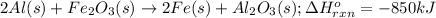
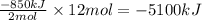 of energy.
of energy.