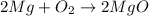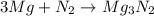Chemistry, 01.07.2019 18:10 jtorres0520
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce mgo. mgo is a white solid, but in these experiments it often looks gray, due to small amounts of mg3n2 , a compound formed as some of the magnesium reacts with nitrogen. write a balanced equation for each reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2


Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to prod...
Questions


Biology, 11.03.2022 01:00

Physics, 11.03.2022 01:00

Mathematics, 11.03.2022 01:00

Social Studies, 11.03.2022 01:00

Mathematics, 11.03.2022 01:00

Mathematics, 11.03.2022 01:00

Mathematics, 11.03.2022 01:00


History, 11.03.2022 01:00

History, 11.03.2022 01:00









Mathematics, 11.03.2022 01:00