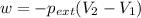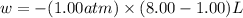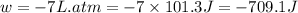Chemistry, 01.07.2019 18:20 jessica2138
One mole of an ideal gas is expanded from a volume of 1.00 liter to a volume of 8.00 liters against a constant external pressure of 1.00 atm. how much work (in joules) is performed on the surroundings? ignore significant figures for this problem. (t = 300 k; 1 l·atm = 101.3 j)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
One mole of an ideal gas is expanded from a volume of 1.00 liter to a volume of 8.00 liters against...
Questions


History, 02.07.2020 22:01


Mathematics, 02.07.2020 22:01



Mathematics, 02.07.2020 22:01



Geography, 02.07.2020 22:01

Chemistry, 02.07.2020 22:01

Mathematics, 02.07.2020 22:01

History, 02.07.2020 22:01

English, 02.07.2020 22:01


History, 02.07.2020 22:01

History, 02.07.2020 22:01

History, 02.07.2020 22:01


Mathematics, 02.07.2020 22:01

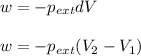
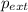 = external pressure = 1.00 atm
= external pressure = 1.00 atm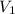 = initial volume of gas = 1.00 L
= initial volume of gas = 1.00 L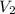 = final volume of gas = 8.00 L
= final volume of gas = 8.00 L