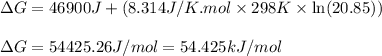Chemistry, 01.07.2019 19:20 elizavlsc4
Co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at 25 ∘c under these conditions: pco2pccl4pcocl2===0.140 atm0.185 atm0.735 atm δg∘f for co2(g) is −394.4kj/mol, δg∘f for ccl4(g) is −62.3kj/mol, and δg∘f for cocl2(g) is −204.9kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Co2(g)+ccl4(g)⇌2cocl2(g) calculate δg for this reaction at 25 ∘c under these conditions: pco2pccl4p...
Questions


Mathematics, 27.01.2021 18:30

Mathematics, 27.01.2021 18:30

Geography, 27.01.2021 18:30




Chemistry, 27.01.2021 18:30

Mathematics, 27.01.2021 18:30





Mathematics, 27.01.2021 18:30






English, 27.01.2021 18:30

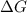 for the reaction is 54.425 kJ/mol
for the reaction is 54.425 kJ/mol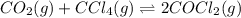
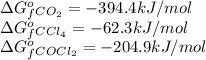
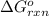 for the reaction, we use the equation:
for the reaction, we use the equation:![\Delta G^o_{rxn}=\sum [n\times \Delta G_f(product)]-\sum [n\times \Delta G_f(reactant)]](/tpl/images/0039/7802/1c133.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(COCl_2)})]-[(1\times \Delta G^o_f_{(CO_2)})+(1\times \Delta G^o_f_{(CCl_4)})]](/tpl/images/0039/7802/08d2c.png)
![\Delta G^o_{rxn}=[(2\times (-204.9))-((1\times (-394.4))+(1\times (-62.3)))]\\\Delta G^o_{rxn}=46.9kJ=46900J](/tpl/images/0039/7802/b07a7.png)
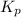 for the given reaction:
for the given reaction: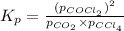
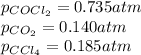
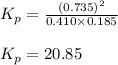
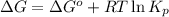
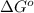 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J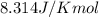
![25^oC=[25+273]K=298K](/tpl/images/0039/7802/df1f6.png)