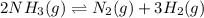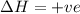Chemistry, 01.07.2019 20:30 ashley8057
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature of the container is raised by 50ºc. which statement below describes a change that will be observed as the system returns to an equilibrium state at the new temperature if δh is positive?
2 nh3(g) ⇄ n2(g) + 3 h2(g)
a. the concentration of ammonia, nh3, will increase.
b. the concentration of nitrogen gas, n2, will decrease.
c. the rate of the forward reaction will increase.
d. there will be no change; increasing the temperature will not change the position of equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

You know the right answer?
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature...
Questions


English, 21.08.2020 07:01

Biology, 21.08.2020 07:01

Advanced Placement (AP), 21.08.2020 07:01


Computers and Technology, 21.08.2020 07:01


Chemistry, 21.08.2020 07:01

Mathematics, 21.08.2020 07:01

Mathematics, 21.08.2020 07:01


History, 21.08.2020 07:01



History, 21.08.2020 07:01

Mathematics, 21.08.2020 07:01

Biology, 21.08.2020 07:01



Advanced Placement (AP), 21.08.2020 07:01