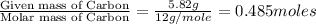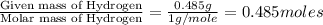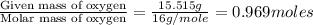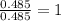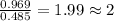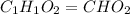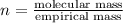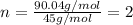Chemistry, 01.07.2019 21:20 robert7248
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 21.33 grams of co2 and 4.366 grams of h2o are produced. in a separate experiment, the molar mass is found to be 90.04 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions










History, 30.03.2020 17:57






Mathematics, 30.03.2020 17:57

Mathematics, 30.03.2020 17:57



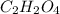
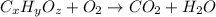
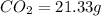
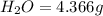
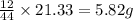 of carbon will be contained.
of carbon will be contained.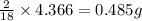 of hydrogen will be contained.
of hydrogen will be contained.