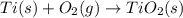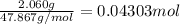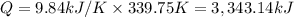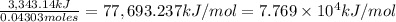The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)<...

Chemistry, 01.07.2019 21:30 faithkristi
The combustion of titanium with oxygen produces titanium dioxide:
ti (s) + o2 (g) → tio2 (s)
when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 91.60 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.
ti = 47.867 amu
o2 = 31.9988 amu
tio2 = 79.8650 amu
report answer in scientific notation use en rather than x 10n

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Questions





Mathematics, 18.07.2019 08:30

Mathematics, 18.07.2019 08:30


History, 18.07.2019 08:30



Mathematics, 18.07.2019 08:30




Mathematics, 18.07.2019 08:30

Spanish, 18.07.2019 08:30


Social Studies, 18.07.2019 08:30


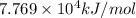 .
.