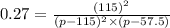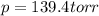Chemistry, 01.07.2019 23:30 NeverEndingCycle
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp = 0.27 at 700 k a reaction mixture initially contains equal partial pressures of no and cl2. at equilibrium, the partial pressure of nocl is 115 torr. what were the initial partial pressures of no and cl2 ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Nitric oxide reacts with chlorine gas according to the reaction: 2 no( g) + cl2( g) ∆ 2 nocl( g) kp...
Questions



Biology, 11.02.2021 01:00







Mathematics, 11.02.2021 01:00

English, 11.02.2021 01:00

History, 11.02.2021 01:00


Mathematics, 11.02.2021 01:00

Social Studies, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Biology, 11.02.2021 01:00

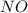 and
and  is 139.4 torr.
is 139.4 torr. 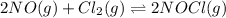
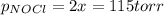
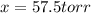
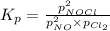
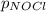 = 2x =115 torr
= 2x =115 torr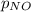 = (p-2x) = p-115 torr
= (p-2x) = p-115 torr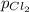 = (p-x)= p-57.5 torr
= (p-x)= p-57.5 torr