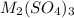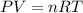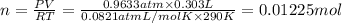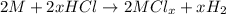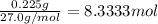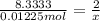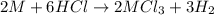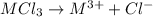Chemistry, 02.07.2019 00:10 naenae6775
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen (measured at 17°c and 741 mmhg) from an excess of hydrochloric acid. deduce from these data the corresponding equation and write formulas for the oxide and sulfate of m.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2
You know the right answer?
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen...
Questions





Social Studies, 15.01.2020 23:31












Computers and Technology, 15.01.2020 23:31



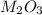 and sulfate of
and sulfate of