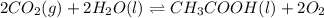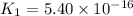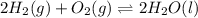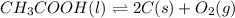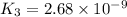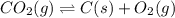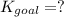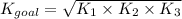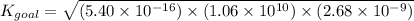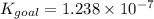Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=? by making use of the following information: 1. 2co2(g)+2h2o(l)⇌ch3cooh(l)+2o2(g), k1 = 5.40×10−16 2. 2h2(g)+o2(g)⇌2h2o(l), k2 = 1.06×1010 3. ch3cooh(l)⇌2c(s)+2h2(g)+o2(g), k3 = 2.68×10−9

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reaction co2(g)⇌c(s)+o2(g), kgoal=?...
Questions

History, 25.03.2021 23:30


English, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30


Mathematics, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30


Social Studies, 25.03.2021 23:30

Geography, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30



Mathematics, 25.03.2021 23:30


History, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30


Computers and Technology, 25.03.2021 23:30

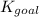 for the final reaction is,
for the final reaction is,