
Chemistry, 02.07.2019 01:10 jen12abc82
Which of the following has the greatest electronegativity difference between the bonded atoms? view available hint(s) which of the following has the greatest electronegativity difference between the bonded atoms? a strong acid made of hydrogen and a halogen, such as hcl a group 1 alkali metal bonded to fluoride, such as lif. carbon bonded to a group 6a (16) nonmetal chalcogen, such as in co a diatomic gas, such as nitrogen (n2).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Which of the following has the greatest electronegativity difference between the bonded atoms? view...
Questions

Geography, 19.03.2021 03:10



History, 19.03.2021 03:10


Mathematics, 19.03.2021 03:10

History, 19.03.2021 03:10


Health, 19.03.2021 03:10


Mathematics, 19.03.2021 03:10




English, 19.03.2021 03:20



Mathematics, 19.03.2021 03:20


Spanish, 19.03.2021 03:20

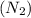 : Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.
: Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.

