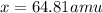Chemistry, 02.07.2019 01:10 guyfromnasa
The average atomic weight of copper, which has two naturally occurring isotopes, is 63.5. one of the isotopes has an atomic weight of 62.9 amu and constitutes 69.1% of the copper isotopes. the other isotope has an abundance of 30.9%. the atomic weight (amu) of the second isotope is amu.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
The average atomic weight of copper, which has two naturally occurring isotopes, is 63.5. one of the...
Questions


History, 20.07.2019 14:30

English, 20.07.2019 14:30

Geography, 20.07.2019 14:30

French, 20.07.2019 14:30


Computers and Technology, 20.07.2019 14:30



Mathematics, 20.07.2019 14:30







History, 20.07.2019 14:30


Mathematics, 20.07.2019 14:30

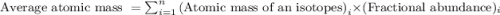 .....(1)
.....(1)![\text{Average atomic mass of copper}=[(62.9\times 0.691)+(x\times 0.309)]](/tpl/images/0040/7303/fa785.png)