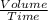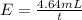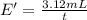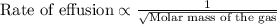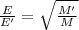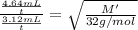Chemistry, 02.07.2019 02:30 slawson4328
Under identical conditions, separate samples of o2 and an unknown gas were allowed to effuse through identical membranes simultaneously. after a certain amount of time, it was found that 4.644.64 ml of o2 had passed through the membrane, but only 3.123.12 ml of of the unknown gas had passed through. what is the molar mass of the unknown gas? unknown molar mass: g/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3


Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Under identical conditions, separate samples of o2 and an unknown gas were allowed to effuse through...
Questions

English, 03.12.2020 21:40

Mathematics, 03.12.2020 21:40

History, 03.12.2020 21:40


English, 03.12.2020 21:40

English, 03.12.2020 21:40

Social Studies, 03.12.2020 21:40

Business, 03.12.2020 21:40




English, 03.12.2020 21:40





Mathematics, 03.12.2020 21:40

English, 03.12.2020 21:40

English, 03.12.2020 21:40