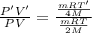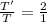Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
Equal volumes of hydrogen and helium gas are at the same pressure. the atomic mass of helium is four...
Questions

Chemistry, 22.06.2019 08:30


Mathematics, 22.06.2019 08:30


Health, 22.06.2019 08:30

Geography, 22.06.2019 08:30

English, 22.06.2019 08:30

History, 22.06.2019 08:30






English, 22.06.2019 08:30

Mathematics, 22.06.2019 08:30





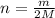
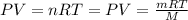 ...(1)
...(1)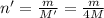
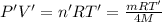 ...(2)
...(2)