

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
Consider the two reactions. 2nh3(g)+3n2o(g)4nh3(g)+3o2(g)⟶4n2(g )+3h2o(l)⟶2n2(g)+6h2o(l) δ∘=−1010 kj...
Questions







Mathematics, 13.06.2020 05:57




Advanced Placement (AP), 13.06.2020 05:57


Mathematics, 13.06.2020 05:57

English, 13.06.2020 05:57

Mathematics, 13.06.2020 05:57

Mathematics, 13.06.2020 05:57

Mathematics, 13.06.2020 05:57



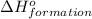 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.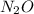 is:
is: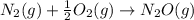
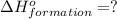
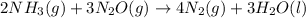
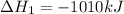 ( ÷ 3)
( ÷ 3)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)![\Delta H^o_{formation}=[\frac{\Delta H_1}{3}]+[\frac{\Delta H_2}{6}]](/tpl/images/0041/0429/5c537.png)
![\Delta H^o_{formation}=[\frac{1010}{3}]+[\frac{1531}{6}]\\\\\Delta H^o_{formation}=591.9kJ](/tpl/images/0041/0429/fba80.png)



