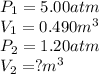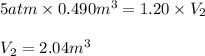Chemistry, 02.07.2019 03:10 ambriyaarmstrong01
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing the gas to drive a piston, increasing the volume of the gas until the pressure drops to 1.20 atm. if the temperature remains constant, what new volume (in m3) does the gas occupy? hint m3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing...
Questions

Mathematics, 01.12.2020 02:10




English, 01.12.2020 02:10


Biology, 01.12.2020 02:10


Chemistry, 01.12.2020 02:10

Mathematics, 01.12.2020 02:10

Biology, 01.12.2020 02:10


Mathematics, 01.12.2020 02:10


Chemistry, 01.12.2020 02:10



Mathematics, 01.12.2020 02:10


Mathematics, 01.12.2020 02:10

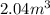
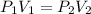 (at constant temperature)
(at constant temperature)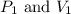 are initial pressure and volume.
are initial pressure and volume.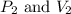 are final pressure and volume.
are final pressure and volume.