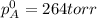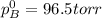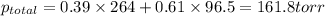Chemistry, 02.07.2019 04:10 NikkiZoeller
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at the same temperature. if 5.50 moles of liquid a and 8.50 moles of liquid b are combined to form an ideal solution, what is the total vapor pressure (in torr) above the solution at 20.0∘c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Liquid a has a vapor pressure of 264 torr at 20∘c, and liquid b has a vapor pressure of 96.5 torr at...
Questions

Social Studies, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00



Chemistry, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00


English, 06.11.2020 06:00

Social Studies, 06.11.2020 06:00

Computers and Technology, 06.11.2020 06:00

Computers and Technology, 06.11.2020 06:10

Biology, 06.11.2020 06:10



Geography, 06.11.2020 06:10



Chemistry, 06.11.2020 06:10

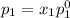 and
and 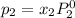
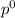 = pressure in the pure state
= pressure in the pure state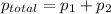
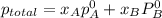
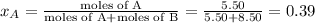 ,
, 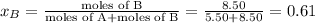 ,
,