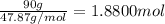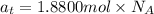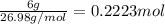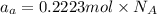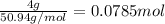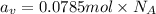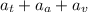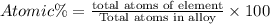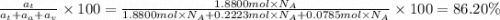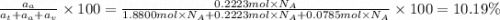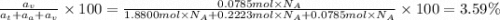Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1


You know the right answer?
The common titanium alloy known as t-64 has a composition of 90 weight% titanium 6 wt% aluminum and...
Questions

Mathematics, 24.01.2021 09:00


Physics, 24.01.2021 09:00


Mathematics, 24.01.2021 09:00

Mathematics, 24.01.2021 09:00

Mathematics, 24.01.2021 09:00


Social Studies, 24.01.2021 09:00

Biology, 24.01.2021 09:00


Mathematics, 24.01.2021 09:00

English, 24.01.2021 09:00

English, 24.01.2021 09:00

Mathematics, 24.01.2021 09:00




Arts, 24.01.2021 09:00

Mathematics, 24.01.2021 09:00