
Chemistry, 02.07.2019 19:10 silviamgarcia
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arrow 2 no(g)+ cl2(g)
calculate the concentrations of all species at equilibrium if
2.0 mol no and 1.0 mol of cl2 are placed in a 1.0 l flask

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1


You know the right answer?
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arro...
2 nocl(g) reverse reaction arro...
Questions

Chemistry, 22.07.2019 20:40



Biology, 22.07.2019 20:40

Social Studies, 22.07.2019 20:40




Mathematics, 22.07.2019 20:40





Health, 22.07.2019 20:40



Social Studies, 22.07.2019 20:40

Spanish, 22.07.2019 20:40


Mathematics, 22.07.2019 20:40

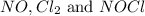 are, 0.05 M, 0.043 M and 0.975 M respectively.
are, 0.05 M, 0.043 M and 0.975 M respectively.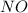 = 2 mole
= 2 mole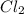 = 1 mole
= 1 mole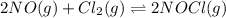
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0043/6110/56950.png)
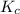 for reverse reaction =
for reverse reaction = 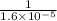
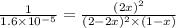
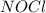 = x M = 0.975 M
= x M = 0.975 M

