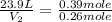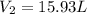Chemistry, 02.07.2019 19:20 azertyqwerty123
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l. the following reaction takes place: n2(g) + 2o2(g)2no2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
Asample of gas contains 0.1300 mol of n2(g) and 0.2600 mol of o2(g) and occupies a volume of 23.9 l....
Questions




Arts, 23.10.2020 22:10

Mathematics, 23.10.2020 22:10

History, 23.10.2020 22:10

English, 23.10.2020 22:10




Mathematics, 23.10.2020 22:10


Mathematics, 23.10.2020 22:10

Mathematics, 23.10.2020 22:10



Law, 23.10.2020 22:10


Social Studies, 23.10.2020 22:10

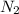 = 0.13 mole
= 0.13 mole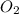 = 0.26 mole
= 0.26 mole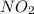 gas.
gas.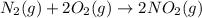
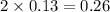 moles of
moles of 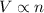
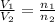
 = initial volume of gas = 23.9 L
= initial volume of gas = 23.9 L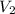 = final volume of gas = ?
= final volume of gas = ?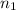 = initial moles of gas = 0.13 + 0.26 = 0.39 mole
= initial moles of gas = 0.13 + 0.26 = 0.39 mole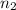 = final moles of gas = 0.26 mole
= final moles of gas = 0.26 mole