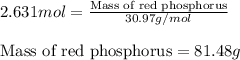Chemistry, 02.07.2019 19:20 fatherbamboo
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes a match. the products of the reaction are tetraphosphorus decoxide and potassium chloride. if 56.0 grams of kcio, are reacted with an excess amount of red phosphorus, how many grams of p0o and kci can be produced? how much red phosphorus is consumed in the reaction? (15 pts) write the balanced reaction first!

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
The reaction between potassium chlorate (kcio,) and red phosphorus (p.) takes place when one strikes...
Questions

Mathematics, 19.01.2021 03:20

Mathematics, 19.01.2021 03:20




Mathematics, 19.01.2021 03:20




English, 19.01.2021 03:20


Health, 19.01.2021 03:20

Mathematics, 19.01.2021 03:20







English, 19.01.2021 03:20

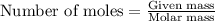 ....(1)
....(1)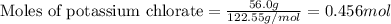
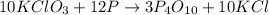
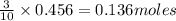 of tetraphosphorus decoxide
of tetraphosphorus decoxide
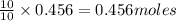 of potassium chloride
of potassium chloride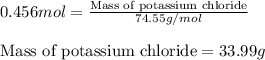
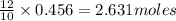 of red phosphorus
of red phosphorus