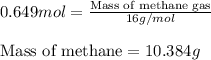Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount of heat transferred when 24.0 g of ch3oh(g) is decomposed by this reaction at constant pressure. b) for a given sample of ch3oh, the enthalpy change during the reaction is 82.1 kj. how many grams of methane gas are produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount...
Questions

Mathematics, 19.04.2021 04:20

Mathematics, 19.04.2021 04:20

Biology, 19.04.2021 04:20



History, 19.04.2021 04:20

Arts, 19.04.2021 04:20

Mathematics, 19.04.2021 04:20


Mathematics, 19.04.2021 04:20

Mathematics, 19.04.2021 04:20



Mathematics, 19.04.2021 04:20



English, 19.04.2021 04:20

Mathematics, 19.04.2021 04:20

Social Studies, 19.04.2021 04:20

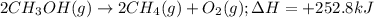
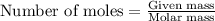 ......(1)
......(1)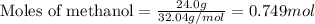
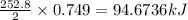
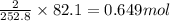 of methane gas is produced.
of methane gas is produced.