
Chemistry, 03.07.2019 20:30 dustonangiecook
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of an unknown hno3 solution required 29.1 ml of 0.200 m ba(oh)2 for complete neutralization. what is the concentration of the hno3 solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of...
Questions



Mathematics, 09.07.2019 13:00

Physics, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00


Business, 09.07.2019 13:00




Mathematics, 09.07.2019 13:00

English, 09.07.2019 13:00


Business, 09.07.2019 13:00


Biology, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00

History, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00

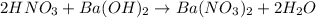
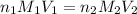
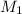 = molarity of
= molarity of 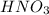 solution = ?
solution = ?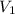 = volume of
= volume of 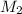 = molarity of
= molarity of 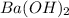 solution = 0.2 M
solution = 0.2 M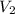 = volume of
= volume of 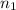 = valency of
= valency of 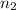 = valency of
= valency of 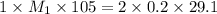
![M_1=0.11M[/tex] Therefore, the concentration of [tex]HNO_3](/tpl/images/0047/6355/af3c6.png) will be 0.11 M.
will be 0.11 M.

