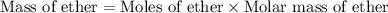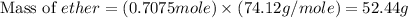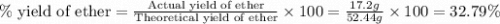Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Diethyl ether is produced from ethanol according to the following equation: 2ch3ch2oh(l) → ch3ch2oc...
Questions





Business, 06.03.2020 20:38


English, 06.03.2020 20:38

Medicine, 06.03.2020 20:38

Computers and Technology, 06.03.2020 20:39





Mathematics, 06.03.2020 20:39


Mathematics, 06.03.2020 20:40

Computers and Technology, 06.03.2020 20:40

English, 06.03.2020 20:40


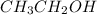 .
.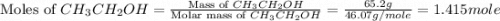
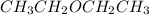
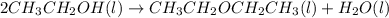
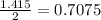 mole of
mole of